Study projects loss of brown macroalgae and seagrasses
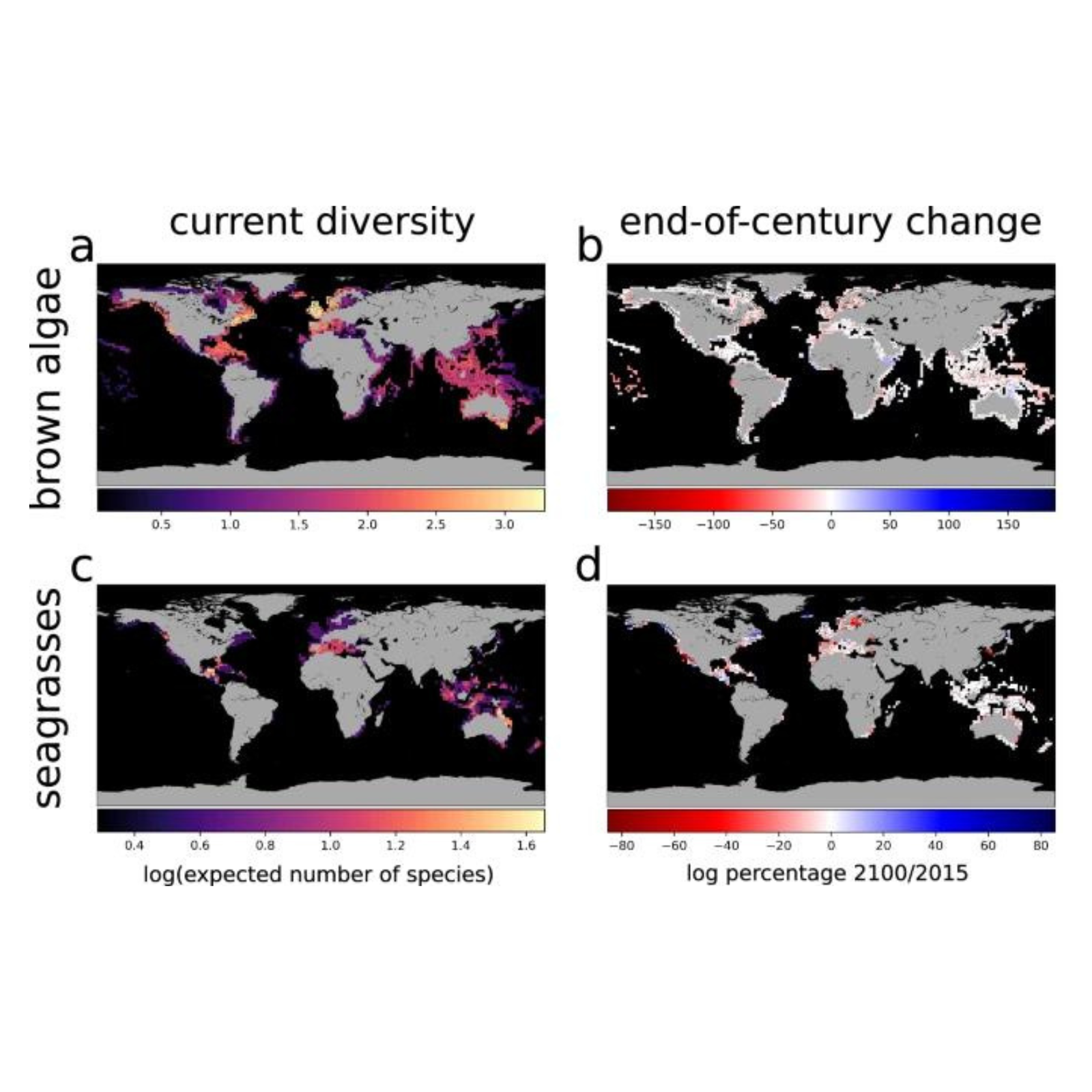
July 1, 2024 Researchers predict that climate change will drive a substantial redistribution of brown seaweeds and seagrasses at the global scale. The projected changes are alarming due to the fundamental role of seaweeds and seagrasses in coastal ecosystems, and provide evidence of the pervasive impacts of climate change on marine life. In a collaborative study between the University of Helsinki and the EU Joint Research Centre, researchers for the first time have modeled the future distribution of brown seaweeds and seagrasses at the global scale. They predict that by 2100, climate change will drive a substantial redistribution of both groups globally: Their local diversity will decline by 3–4% on average and their current distribution will shrink by 5–6%. More notably, the preferred habitat for both brown seaweeds and seagrasses will undergo a substantial global reduction (78–96%) and will shift among marine regions, with potential expansions into Arctic and Antarctic regions. The research is published in the journal Nature Communications. “We find it alarming that coastal areas worldwide will become dramatically less hospitable for habitat-forming macrophytes, as this might have severe and widespread impacts on coastal ecosystem functioning at the global scale. Interestingly, while global percentual declines in diversity show similar trends for seagrasses and brown macroalgae, the regional patterns are strikingly different between the two groups,” says Federica Manca, the lead author of the study from the University of Helsinki. Present distribution and projected end-of-century changes in global macrophyte species diversity. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-48273-6 Why should we care about seaweeds and seagrasses? Brown seaweeds and seagrasses provide important ecological and socio-economic services in coastal areas worldwide. They support coastal biodiversity and fisheries, ensure coastal protection, participate in ocean nutrient recycling, contribute to carbon sequestration and climate change mitigation. As climate change is severely threatening macrophyte habitats and the services they provide, we urgently need to understand how both brown seaweeds and seagrasses will respond to changing climatic conditions in the coming decades. Previous studies have modeled the future distribution of these habitat-forming macrophytes, focusing on regional or local scales only and on a limited number of species. In contrast, this study is the first to provide a comprehensive view of the effects of climate change on more than 200 species of brown seaweeds and seagrasses at the global scale. The results show that the redistribution of these habitat-forming marine macrophytes will be geographically heterogeneous, and highlight the regions where the loss of macrophyte diversity and habitat will be most severe, such as the Pacific coast of South America for brown seaweeds, and the coast of Australia for seagrasses. Additionally, researchers have identified macrophyte species that will be more severely affected by climate change, like the Atlantic seaweed Laminaria digitata. The findings can help identify target areas and species for conservation, potentially buffering the impact of climate change. Surprisingly, and contrary to expectations, the models did not predict severe losses of brown seaweed or seagrass diversity in the tropics but rather at intermediate and high latitudes, such as along the Atlantic coasts of Europe and in the Baltic Sea. This indicates that end-of-century climatic conditions in these regions might exceed the tolerance limits of resident macrophyte species. The Baltic Sea is at the forefront in the rate at which climate change is influencing the ecosystem. “Combined with a legacy of multiple other disturbances (such as eutrophication) and low species diversity with only a few brown seaweeds and seagrasses, the Baltic Sea is exceptionally vulnerable to these predicted changes,” says Alf Norkko, professor at the Tvärminne Zoological Station, University of Helsinki. “Another surprising—and alarming—result is the dramatic loss of highly suitable habitat for both macroalgae and seagrasses globally: Coastal areas worldwide will become substantially less hospitable for habitat-forming macrophytes,” adds Dr. Mar Cabeza from the Global Change and Conservation Group at the University of Helsinki. The disappearance of these habitat-forming macrophytes can trigger cascading effects on other species, compromising the integrity of entire ecosystems and undermining ecological and socio-economic services important to human society. Thus, forecasting changes in the distribution of habitat-forming species is crucial to raise awareness of climate change impacts and foster conservation efforts accordingly. “Our findings confirm, once again, that climate change might have profound impacts on ecosystems, promoting rapid and most often detrimental changes to the diversity and resilience of natural communities. In fact, habitat-forming macrophytes support biodiversity through an exceptional diversity of ecological interactions.” “Hence, their projected loss and redistribution might lead to unpredictable cascading effects, most likely resulting in the local extinction of many associated species,” says Giovanni Strona from the EU Joint Research Centre. More information: Federica Manca et al, Projected loss of brown macroalgae and seagrasses with global environmental change, Nature Communications (2024). DOI: 10.1038/s41467-024-48273-6 This article is republished from PHYS.ORG and provided by the University of Helsinki.
Seagrass clone in the Baltic sea is more than 1,400 years old
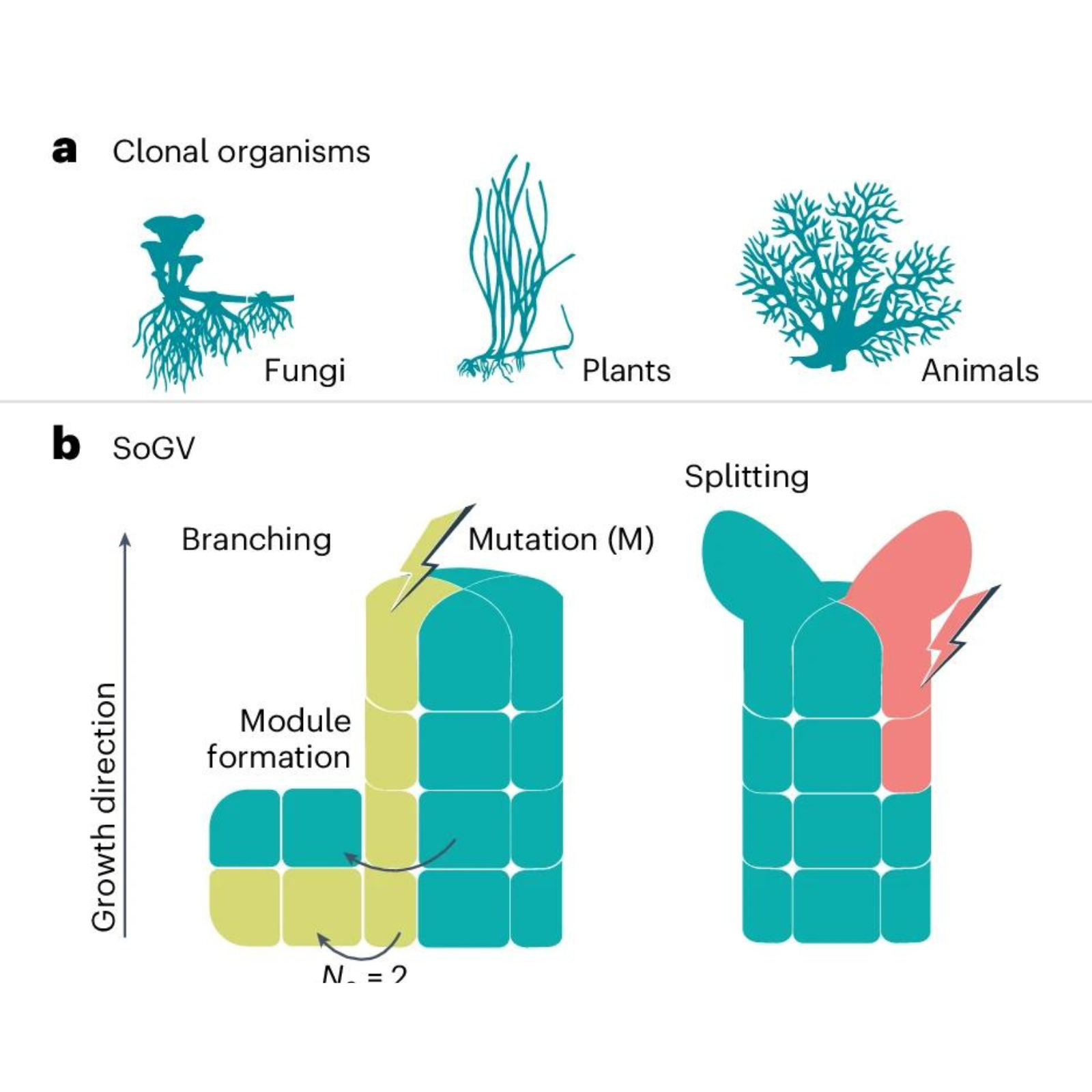
June 24, 2024 Using a novel genetic clock, a team of researchers from Kiel, London, Oldenburg, and Davis, California, has determined the age of a large marine plant clone for the first time. This seagrass clone from the Baltic Sea dates back to the migration period 1,400 years ago. The newly developed clock can be applied to many other species, from corals and algae to plants such as reeds or raspberries. The scientists have published their work in the journal Nature Ecology and Evolution. “Vegetative reproduction as an alternative mode of reproduction is widespread in the animal, fungal, and plant kingdoms,” explains research leader Dr. Thorsten Reusch, Professor of Marine Ecology at the GEOMAR Helmholtz Centre for Ocean Research Kiel. These so-called “clonal species” produce genetically similar offspring by branching or budding and often reach the size of a football field or more. However, these offspring are not genetically identical. (a), Multicellular clonal species exist across the tree of life. (b), Allele frequency change of SoGV due to the formation of new modules by branching or splitting. A new module is initiated either directly by the stem cells (that is, splitting) or by the daughter cells of the stem cells (that is, branching). Splitting reduces the size of the original stem cell population, while branching leaves the original cell population untouched. During the formation of new modules, the cell population undergoes a genetic bottleneck. c,d, The accumulation rate of fixed SoGV is independent of module formation rate. The tree topology depicts a module undergoing (multiple) module formation events, where the dashed line and the solid line represent the original module and the new module respectively. New mutations (M) occur at a constant rate, and only mutations in the new modules are depicted (with a different colour). For each timepoint, the vertical length of the colours represents the frequency of the SoGV within the module. Clonal dynamics in a single module (solid line in tree structure) are depicted as a Muller plot that shows the nested allele frequency of SoGV over time. The frequency of SoGV changes during module formation events, due to the bottleneck. Eventually, SoGVs are either fixed or lost. Under low module formation rate (c), fixation events are rare. Thus, many SoGVs have accumulated in the intervening time and are fixed simultaneously. Under high module formation rate (d), fixation events occur more frequently, but with fewer SoGVs fixed at each branching event. CREDIT: Nature Ecology & Evolution (2024). DOI: 10.1038/s41559-024-02439-z Previous work by a team led by GEOMAR researchers had already shown that somatic mutations accumulate in vegetative offspring, a process similar to cancer. Now, a team led by Prof. Dr. Reusch, Dr. Benjamin Werner (Queen Mary University London, QMUL), and Prof. Dr. Iliana Baums (Helmholtz Institute for Functional Marine Biodiversity at the University of Oldenburg, HIFMB) has used this mutation accumulation process to develop a novel molecular clock that can determine the age of any clone with high precision. Researchers at the University of Kiel, led by Professor Reusch, applied this novel clock to a worldwide dataset of the widespread seagrass Zostera marina (eelgrass), ranging from the Pacific to the Atlantic and the Mediterranean. In Northern Europe in particular, the team found clones with ages of several hundred years, comparable to the age of large oak trees. The oldest clone identified was 1402 years old and came from the Baltic Sea. This clone reached this advanced age despite a harsh and variable environment. This makes the eelgrass clone older than the Greenland shark or the Ocean Quahog, which live only a few hundred years. These new age and longevity estimates for clonal species fill an important knowledge gap. Particularly in marine habitats, many fundamental habitat-forming species such as corals and seagrasses can reproduce vegetatively, and their clones can become very large. The continuous production of small, genetically identical but physically separated shoots or fragments from the parent clone means that age and size are decoupled in these species. The new study now provides a tool to date these clones with high accuracy. “Such data are, in turn, a prerequisite for solving one of the long-standing puzzles in conservation genetics, namely why such large clones can persist despite variable and dynamic environments,” says Reusch. Once a high-quality eelgrass genome was available, work could begin. Another key factor in the study was that colleagues at the University of California, Davis (UC Davis) had kept a seagrass clone in their culture tanks for 17 years, which served as a calibration point. “This paper shows how interdisciplinary interactions between cancer evolutionary biologists and marine ecologists can lead to new insights,” says Dr. Werner, Lecturer in Mathematics and Cancer Evolution at QMUL, who focuses on the somatic evolution of tumors which also develop clonally. Prof. Dr. Baums, molecular ecologist at the HIFMB, adds, “We can now apply these tools to endangered corals to develop more effective conservation measures, which we urgently need as unprecedented heat waves threaten coral reefs.” “We expect that other seagrass species and their clones of the genus Posidonia, which extend over more than ten kilometers, will show even higher ages and thus be by far the oldest organisms on Earth,” says Reusch. These will be the next objects of study. More information: Lei Yu et al, A somatic genetic clock for clonal species, Nature Ecology & Evolution (2024). DOI: 10.1038/s41559-024-02439-z This article is republished from PHYS.ORG and provided by Helmholtz Association of German Research Centres
Seagrass meadows expanding near inhabited islands in Maldives

Swimming through the crystal clear waters of the Maldives, a nation renowned for its marine life, it could be easy to forget that these delicate ecosystems stand on the frontline of climate change and that seagrass habitats are in crisis globally. Now, my research, which combined hundreds of hours of fieldwork with thousands of satellite images, has uncovered something unexpected: Maldivian seagrasses have expanded three-fold over the last two decades—and island populations could be playing a part. I also discovered that seagrass is surprisingly three times more likely to be found next to inhabited islands, rather than uninhabited. So this flowering plant seems to benefit from living in seas close to humans. Seagrass habitats are expanding in some areas, to the surprise of researchers. CREDIT: Matthew Floyd, CC BY-ND Seagrasses grow along coasts all around the world. They can help guard against climate change yet they are frequently underappreciated. In the Maldives, seagrass meadows are dug up to maintain the iconic white beaches that are a frequent feature of honeymoon photos. Important marine habitats have declined in the Maldives. Amid this backdrop of environmental uncertainty, I have spent more than three years studying seagrasses here alongside a team of scientists. We found that seagrasses are faring remarkably well and one of the most plausible drivers could be the supply of nutrients from densely populated areas, such as tourist resorts. Every day, human activities could provide valuable nutrients for seagrass habitats in an otherwise nutrient limited environment. Food waste is traditionally discarded into the sea from the beach and rain can wash excess fertilizers from farmland into the ocean. As human populations and fertilizer use have both increased, we suspect that seagrass meadows have started to thrive and expand as a result of this increased nutrient supply. Additionally, building work around islands may create more suitable habitats for seagrass. Land reclamation is widespread across the country as the population has expanded by 474% since 1960. (A) Trends in overall seagrass extent in the Maldives from 2000-2021, (B) Seagrass area trends detailing changes across all 26 atolls from 2000-2021 CREDIT: Matthew Floyd, CC BY-ND During this development, sand is dug up from the seabed and some inevitably spills into the water. The structure of seagrass meadows can slow down local water currents, promoting suspended sand grains to sink and creating more sediment for future generations of seagrass to grow into. Currently, nutrient inputs seem to be creating just the right conditions for seagrasses. But if nutrients continue to increase, there is a risk that the seagrasses will be outcompeted by seaweeds and smothered. Continued land reclamation works that disregard seagrass may also remove this important habitat. So the future of this Maldivian success story may therefore largely lie in our hands. The ecotourism paradox Although seagrass removal has done little to curb habitat expansion, it highlights a troubled relationship with the tourism industry upon which so many jobs in the Maldives depend. Because it can ultimately make water depths shallower, seagrass can limit boat access and mooring, and therefore interfere with daily life. The proliferation of seagrass in areas of domestic refuse has understandably damaged its image in the eyes of the public. But, by making coastal waters shallower, seagrasses reinforce coastal protection. And by growing close to refuse sites, they absorb excess nutrients and clean the water of pathogens. Despite being a vital tool in the fight against climate change, seagrass clearly has an image problem on the islands. As a marine ecologist, I firmly believe that conservation scientists—and ecotourists—have an important role to play in conveying the value of seagrasses. Conservationists must also fully appreciate the challenges that meadow expansion can bring to local communities, and understand how the needs of conservation and tourism may differ. There is hope. A campaign called #ProtectMaldivesSeagrass, recently launched by Blue Marine Foundation and Maldives Underwater Initiative, led to 37 resorts (out of a total of 168) pledging to protect their seagrass meadows. Additionally, the data from my research can be used to protect seagrass habitats and quantify their value to people and nature. Hopefully, the unexpected—yet welcome—success of seagrass in the Maldives is a cause for conservation optimism. And perhaps tourist resorts can learn to love their newly expanding neighbors. More information: Floyd, M., East, H.K., Traganos, D. et al. Rapid seagrass meadow expansion in an Indian Ocean bright spot. Sci Rep 14, 10879 (2024). https://doi.org/10.1038/s41598-024-61088-1 This article is republished from The Conversation under a Creative Commons license. Read the original article.
Making seagrass restoration more resistant to rising temperatures

New research demonstrates that seagrass habitat restoration can be enhanced by including other grasses in addition to the declining or lost species and – ultimately – that restoration efforts must proactively select species that can withstand current and intensifying stressors driven by human activities and climate change. Rising global temperatures combined with centuries of humans working within our seascapes has reshaped coastal ecosystems. Rebuilding or restoring coastal habitat is becoming a top priority for natural resource conservation and as an insurance policy for the provision of critical services including shoreline protection, clean water, and seafood. Yet, successful habitat restoration is still rare, and most efforts are unsustainably expensive and labor intensive. “Any gardener knows the difficultly in mastering how to grow a plant from seed or a clipping, and the same goes for restoration practitioners using habitat-forming species – discovering the perfect conditions.” says Enie Hensel, lead-author, postdoctoral researcher at UF IFAS SWES Nature Coast Biological Station and former postdoctoral researcher at William & Mary’s Virginia Institute of Marine Science (VIMS). TOP LEFT IMAGES ARE COLLECTED WIDGEONGRASS (TOP) AND EELGRASS (BOTTOM) SEEDS THAT WERE SEEDED IN LYNNHAVEN RIVER, VIRGINIA BEACH, VA TO RESTORE SEAGRASS HABITAT (SECOND FROM LEFT) THAT CAN HANDLE MULTIPLE NOVEL STRESSORS INCLUDING WARMING TEMPERATURES. CREDIT: CREDIT: ENIE HENSEL, UMCES IAN SYMBOLS, VIRGINIA INSTITUTE OF MARINE SCIENCE. Seagrasses are experiencing global declines and, in the Chesapeake Bay, United States, where this study was conducted, intensifying heat waves are a main cause for declines in the dominant seagrass, eelgrass (Zostera marina). Recent research has shown the most successful restorations span large areas and location selection is key. The location should have a certain sand or sediment type, water quality level, temperature range, and the presence of beneficial ‘bugs’ or invertebrates that graze off grass-smothering algae – all of which are dependent on grass identity (M. M can Katwijk et al., 2016). “These novel environmental conditions are a challenge for restoration. But what tends to always thrive in someone’s yard, no matter how hard one caters to their prized lawn grass, are weeds. And this fact might translate well to seagrass restoration – incorporating ‘weedy,’ or generalist, seagrass species that aren’t necessarily the targeted species for a given restoration,” says Enie. “This project is important because Chesapeake Bay eelgrass has been declining for the past 30 years due to warming waters and we need to start thinking about alternative restoration strategies that accommodate this shifting environmental baseline. Here, we included a widely distributed generalist seagrass, widgeongrass [Ruppia maritima], which increases the portfolio of species diversity, providing some insurance that helps enhance long term restoration success.” says co-author Christopher J. Patrick, co-lead of the Submerged Aquatic Vegetation Restoration portion of the U.S. Army Corps of Engineer’s Lynnhaven River Basin Ecosystem Restoration Project and director of the Chesapeake Bay’s SAV Monitoring and Restoration Program For this study, Enie and her collaborators leveraged a planned seagrass restoration in the lower Chesapeake Bay and conducted a field experiment to evaluate (1) which seeding methods yielded the most widgeongrass growth, tested if seeding widgeongrass next to eelgrass can increase restoration success, and quantified how either seagrass species changes restored bed structure, invertebrate communities, and nitrogen cycling. In the following year, researchers operationalized their experimental findings during a multi-acre pilot restoration. “This is the first large-scale restoration effort in the lower Chesapeake Bay to use widgeongrass, and one of the few field experiments to identify how to best grow widgeongrass in the wild,” Enie says. In the experiment, hand-seeded widgeongrass successfully grew. The highest survival and growth were when seeding mimicked nature and for this study that meant seeding widgeongrass in the fall with no pre-seed treatment – an advantageous finding for practitioners as it requires the least effort. Additionally, by seeding both widgeongrass and eelgrass, the restoration nearly doubled in size as widgeongrass was seeded in the shallows where water temperatures were above local practitioners’ recommendation for eelgrass restorations. “Two exciting findings: these young widgeongrass beds were, one, full of epiphytic algal grazers, the ‘beneficials’ for a seagrass meadow and two, recycled less nitrogen than its surrounding sandy substrate. While this trend will likely change as widgeongrass matures, young widgeongrass recycling a negligible amount of nitrogen in a nutrient-rich area should have a positive effect on both grasses by not further increasing available nutrients, a known seagrass stressor in human-influenced systems like the Virgina Beach area” says Enie. This study was a part of the initial phase for SAV Restoration led and supported by William G. Reay of the Chesapeake Bay National Estuarine Research Reserve (NOAA NOS/OCM, NA21NOS4200127) at VIMS as well as Robert J. Orth and Christopher J. Patrick of VIMS (NSF OCE 1737258 and 1658135) for the ‘Lynnhaven River Basin Ecosystem Restoration Project’ led and funded by the U.S. Army Corps of Engineers with local Norfolk District USACE (W912HZ-20-2-0021) as well as collaboration with City of Virginia Beach Department of Defense. More information: Hensel et al, Incorporating generalist seagrasses enhances habitat restoration in a changing environment , Journal of Applied Ecology (2024). DOI: 10.1111/1365-2664.14643 This story is republished courtesy of Virginia Institute of Marine Science.
Seagrass production around artificial reefs is resistant to human stressors

Artificial reefs might help to restore the ocean’s ability to fight against climate change. The reefs boost the productivity of seagrass meadows by attracting fish, which can improve the ability of these habitats to lock up more carbon dioxide beneath the waves. Breeze blocks placed in one of the ocean’s most endangered habitats provide an unexpected lift for fish. Seagrass meadows are found across the world, reaching from the tropics up into the lower reaches of the Arctic circle. They are incredibly valuable habitats, providing a nursery for young fish as well as sucking vast quantities of carbon dioxide from the atmosphere. However, with an area of seagrass the size of a football pitch being lost every 30 minutes, it’s more important than ever to find out how to turn things around. A new study in the Caribbean has shown that artificial reefs can help to bolster their growth in the tropics, even as threats such as fishing and nutrient pollution continue. Dr Jacob Allgeier, a co-author of the paper, says, ‘By attracting fish, whose faeces provide concentrated nutrients for the seagrass, the artificial reefs increase the primary production of the entire ecosystem.’ ‘We are now investigating how this cascades up the food web. The new energy has to go somewhere, so we are quantifying how it affects invertebrates and fish with our evidence suggesting that it is fuelling increases in both.’ The findings of the study were published in Proceedings of the Royal Society B. Artificial reefs and seagrass One of the biggest issues affecting seagrass is nutrient pollution, often from the release of human sewage. While the influx of nutrients can initially boost the growth of the meadows, it also promotes the growth of algae which reduces the amount of sunlight getting to the seagrass and harms it in the long run. Alongside fishing which causes levels of the fish faeces that fertilise the meadows to drop, it was thought that the combination of these two issues might work in unexpected ways to hinder the growth of seagrass. But the current study has revealed some surprising results. It has found that the productivity of seagrass in both disturbed and undisturbed meadows was increased by the presence of an artificial reef, while algae didn’t actually seem to pose an issue, even in areas where nutrient pollution was high. Mona Andskog, the PhD student who led the research, explains, ‘Artificial reefs built in seagrass create a positive feedback loop. They attract fish that use the reefs for shelter which, in turn, supply new nutrients from their faeces that fertilise the seagrass around the reef.’ ‘This increased primary production can increase invertebrate production by providing more food and shelter for invertebrates, which in turn provide more food for fishes.’ Experiments in Haiti, at some of the most fished sites included in the study, also showed that the artificial reefs were providing additional benefits to the fish. Large numbers of small fish were found at the site because of the difficulty in using nets around the reef, meaning that the overall biomass of fish was at times larger than in unfished areas measured elsewhere in the study. While artificial reefs present a promising option for tropical seagrasses, they’re likely to have a much more limited impact on temperate meadows. These waters already tend to have higher nutrient levels, meaning that any contribution the reef would made to overall growth would be small. The scientists now hope to explore how the placing of artificial reefs can affect seagrass ecosystems, as well as expanding their research to the Dominican Republic. ‘We will be testing how different configurations of artificial reef clusters can affect the production and fish community composition,’ Jacob says. ‘This includes the number of artificial reefs in each cluster, as well as their arrangement.’ ‘As with this research, we hope to simultaneously use the reefs to test fundamental questions about production in these highly impacted ecosystems as well as optimising the positive feedback that is initiated by the artificial reefs.’ More information: Mona A. Andskog et al, Seagrass production around artificial reefs is resistant to human stressors, Proceedings of the Royal Society B: Biological Sciences (2023). DOI: 10.1098/rspb.2023.0803 This story is republished courtesy of Natural History Museum. Read the original story here.
How eelgrass spread around the world
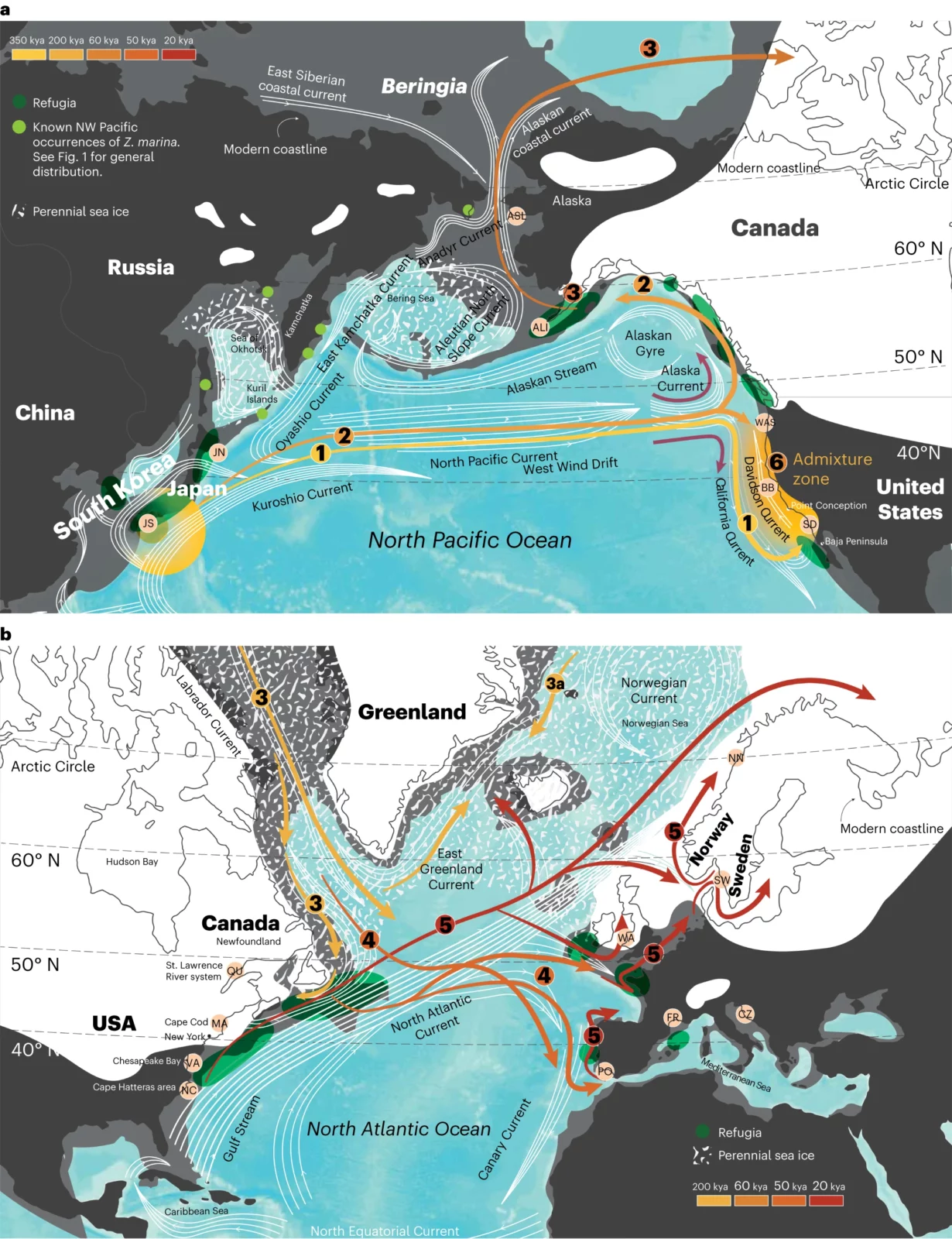
Seagrasses evolved from freshwater plants and use sunlight and carbon dioxide (CO2) for photosynthesis and are able to thrive in depths down to 50 meters. In contrast to algae, they possess roots and rhizomes that grow in sandy to muddy sediments. The grass-like, leaf-shoots produce flowers and complete their life cycle entirely underwater. Seeds are negatively buoyant but seed-bearing shoots can raft, thus greatly enhancing dispersal distances at oceanic scale. As a foundational species, eelgrass (Zostera marina) provides critical shallow-water habitats for diverse biotas and also provides numerous ecosystem services including carbon uptake. Seagrasses have recently been recognized as one of the important nature-based contributions to store carbon in the ocean. The sediment below seagrass meadows can sequester between 30 and 50 times more carbon annually that the roots of forests on land. Unfortunately, the continuing loss of seagrass beds worldwide—including eelgrass—is of acute concern. An international group of researchers, including Richard Unsworth and coordinated by Professor Thorsten Reusch, Head of the Research Division Marine Ecology at GEOMAR Helmholtz Centre for Ocean Research Kiel, used complete nuclear and chloroplast genomes from 200 individuals and 16 locations to reconstruct and date the colonisation history of the eelgrass Zostera marina from its origin in the Northwest Pacific Ocean to the Pacific, Atlantic and the Mediterranean. The findings described in an article and a Research Briefing published in Nature Plants beg the question, “How well will eelgrass adapt to our new, rapidly changing climate?” Using a phylogenomic approach the scientists were able to determine that Z. marina first arose in the Japanese Archipelago region and then crossed the Pacific from west to east in at least two colonisation events, probably supported by the North Pacific Current. The scientists then applied two DNA “molecular clocks”—one based on the nuclear genome and one based on the chloroplast genome—to deduce the time when eelgrass populations diverged into new ones. The DNA mutation rate was calculated and calibrated against an ancient, whole genome duplication that occurred in eelgrass. Both nuclear and chloroplast genomes revealed that eelgrass dispersed to the Atlantic through the Canadian Arctic about 243 thousand years ago. This arrival is far more recent than expected—thousands of years versus millions of years, as is the case with most Atlantic immigrant species during the Great Arctic Exchange some 3.5 million years ago. Reusch explains, “We thus have to assume that there were no eelgrass-based ecosystems—hotspots of biodiversity and carbon storage—in the Atlantic before that time. Recency was also mirrored in an analysis of the associated faunal community, which features many fewer specialized animals in the Atlantic as compared to the Pacific eelgrass meadows. This suggests that there was less time for animal-plant co-evolution to occur.” Mediterranean populations were founded from the Atlantic about 44 thousand years ago and survived the Last Glacial Maximum. By contrast, today’s populations found along the western and eastern Atlantic shores only (re)expanded from refugia after the Last Glacial Maximum, about 19 thousand years ago—and mainly from the American east coast with help from the Gulf Stream. In addition, the researchers further confirmed the huge difference in genomic diversity between the Pacific and Atlantic, including latitudinal gradients of reduced genetic diversity in northern populations. “Both Atlantic compared to Pacific populations, and northern versus southern ones are less diverse on a genetic level than their ancestors by a factor of 35 among the most and least diverse one,” said postdoctoral scientist Dr. Lei Yu, first author of the publication, which was a chapter in his doctoral thesis. “This is due to bottlenecks arising from past ice ages, which raises concerns as to how well Atlantic eelgrass, will be able to adapt to climate change and other environmental stressors based on its genetic capacity.” “Warming oceans have already caused losses of seagrass meadows at the southern range limits, in particular North Carolina and southern Portugal. In addition, heat waves have also caused losses in shallow waters in some the northern parts of the distribution,” noted Reusch. “This is not good news because seagrass meadows form diverse and productive ecosystems, and no other species is able to take on the role of eelgrass if meadows cannot persist under future conditions.” “One possibility for restoration might be to borrow some genetic diversity from Pacific eelgrass to fortify diversity in the Atlantic. Our next step is to interrogate the eelgrass pangenome. A new reference genome from Pacific eelgrass is currently under development and should tell us more about the adaptive ecotypic capacity across its global range of habitats,” said Prof. Jeanine Olsen, emeritus professor from the University of Groningen who initiated the study and coordinated the work between the Joint Genome Institute (JGI) and the research team. More information: Yu, L. etal, Ocean current patterns drive the worldwide colonization of eelgrass (Zostera marina), Nature Plants (2023). DOI: 10.1038/s41477-023-01464-3 Story provided by Helmholtz Association of German Research Centres
Green sea turtles have traveled to the same seagrass to eat for 3,000 years

For approximately 3,000 years, generations of green sea turtles have returned to the same seagrass meadows to eat. This was discovered by Willemien de Kock, a historical ecologist at the University of Groningen, by combining modern data with archaeological findings. Sea turtles migrate between specific breeding places and eating places throughout their lives–this much was known. But the fact that this stretches over many generations highlights the importance of protecting seagrass meadows along the coasts of North Africa. The results were published in PNAS on July 17. When young green sea turtles hatch, their parents have already left for a long journey. The little turtles clumsily make their way off the beach into the ocean and, not yet able to navigate the long migration of their parents, float around for years. During this time, they are not very picky eaters, omnivores even. Then, at about five years of age, they swim to the same area where their parents went, to eat a herbivore’s diet of seagrass. Along the coasts of the eastern Mediterranean Sea, volunteers are active to protect the nests of the endangered green sea turtles. However, as Willemien de Kock explains, “We currently spend a lot of effort protecting the babies but not the place where they spend most of their time: the seagrass meadows.” And crucially, these seagrass meadows are suffering from the effects of the climate crisis. Analyzing sea turtle bones In the attic of the Groningen Institute of Archaeology at the University of Groningen, De Kock had access to boxes full of sea turtle remains from archaeological sites in the Mediterranean Sea area. The excavations were already done by her supervisor, Dr. Canan Çakırlar. “All I had to do was dig in some boxes,” De Kock says. By analyzing the bones, De Kock was able to distinguish two species within the collection of bones: the green sea turtle and the loggerhead turtle. De Kock was also able to identify what the sea turtles had been eating. This relied on a substance called bone collagen. By inspecting the bone collagen with a mass spectrometer, De Kock could detect what kind of plants the sea turtles must have eaten. “For instance,” De Kock explains, “one plant might contain more of the lighter carbon-12 than another plant, which contains more of the heavier carbon-13. Because carbon does not change when it is digested, we can detect what ratio of carbon is present in the bones and infer the diet from that.” Combining old and new Modern satellite tracking data from the University of Exeter then provided De Kock with information on the current traveling routes and destinations of sea turtles. Researchers from Exeter had also been taking tiny samples of sea turtles’ skins, which revealed similar dietary information as De Kock found in bones. De Kock was, therefore, able to draw conclusions, connecting diets of millennia ago to specific locations. She found that for approximately 3,000 years, generations of green sea turtles have been feeding on sea grass meadows along the coasts of Egypt and West Libya. The results for loggerhead turtles were less specific because they had a more varied diet. So, why is it relevant to know the eating habits of a species over many past generations? Because we collectively suffer from the shifting baseline syndrome: slow changes in a larger system, such as an animal population, go unnoticed because each generation of researchers redefines what the natural state was, as they saw it at the start of their careers. “Even long-term data goes back only about 100 years,” says De Kock. “But tracing back further in time using archaeological data allows us to better see human-induced effects on the environment. And it allows us to predict, a bit.” In fact, recent models have shown a high risk of widespread loss of seagrass in precisely these spots where green sea turtles have been going for millennia. This could be detrimental to the green sea turtle, precisely because of its high fidelity to these places. More information: de Kock, Willemien, Threatened North African seagrass meadows have supported green turtle populations for millennia, Proceedings of the National Academy of Sciences(2023). DOI: 10.1073/pnas.2220747120 Story provided by University of Groningen
Caribbean seagrasses provide services worth $255B annually, including vast carbon storage

Discussions of valuable but threatened ocean ecosystems often focus on coral reefs or coastal mangrove forests. Seagrass meadows get a lot less attention, even though they provide wide-ranging services to society and store lots of climate-warming carbon. But the findings of a new University of Michigan-led study show that seagrass ecosystems deserve to be at the forefront of the global conservation agenda, according to the authors. It’s the first study to put a dollar value on the many services—from storm protection to fish habitat to carbon storage—provided by seagrasses across the Caribbean, and the numbers are impressive. Using newly available satellite data, the researchers estimate that the Caribbean holds up to half the world’s seagrass meadows by surface area, and it contains about one-third of the carbon stored in seagrasses worldwide. They calculated that Caribbean seagrasses provide about $255 billion in services to society annually, including $88.3 billion in carbon storage. In the Bahamas alone, the ecosystem services provided by seagrasses are valued at more than 15 times the country’s 2020 gross domestic product, according to the study published online June 21 in the journal Biology Letters. “Our study is the first to show that seagrass beds in the Caribbean are of global importance in their areal extent, in the amount of carbon they store, and in the value of the economic services they provide to society,” said study lead author Bridget Shayka, a doctoral student in the U-M Department of Ecology and Evolutionary Biology. “The findings underscore the importance of conserving and protecting these highly threatened and globally important ecosystems, which are critical allies in the fight against climate change.” One way to prioritize seagrass conservation would be to include those verdant undersea meadows in global carbon markets through projects that minimize loss, increase areal extent or restore degraded beds. The idea of selling “blue carbon” offset credits, which monetize carbon stored in coastal and marine ecosystems, is gaining traction for several reasons. For one, many island nations that have already been impacted by climate change—through increasingly intense hurricanes or rising sea levels, for example—have large areas of valuable coastal ecosystems that store carbon and that provide other services to society. Blue carbon (the name refers specifically to carbon stored in coastal and open-ocean ecosystems while “green carbon” refers more broadly to carbon stored in all natural ecosystems) offset credits could be a way for wealthier countries to compensate for their contribution to human-caused climate change while at the same time benefiting the economies of impacted countries and helping to conserve coastal ecosystems, which are among the most impaired in the world. Threats to seagrass meadows include coastal development, chemical pollution, recreation, shipping and climate change. “Because seagrass ecosystems are both highly important for carbon storage and sequestration, and are highly degraded globally, they represent an important burgeoning market for blue carbon,” said marine ecologist and study senior author Jacob Allgeier, an associate professor in the U-M Department of Ecology and Evolutionary Biology. “Yet, to date, a fundamental impediment to both evaluating seagrass and promoting it in the blue carbon market has been the lack of thorough seagrass distribution data.” For their study, the U-M-led team used newly available seagrass distribution data collected by the PlanetScope constellation of small DOVE satellites. They classified Caribbean seagrass ecosystems as either sparse or dense and estimated the amount of carbon in plants and sediments using data from Thalassia testudinum, the dominant seagrass species in the region. The researchers then calculated a conservative economic value for the total ecosystem services provided by seagrasses in the Caribbean and for the stored carbon, using previously published estimates for the value of services including food production, nursery habitat for fishes and invertebrates, recreation and carbon storage. Grouper, queen conch and lobster are among the commercially harvested animals that rely on Caribbean seagrass. Green sea turtles, tiger sharks and manatees also depend on it. To estimate the dollar value of the carbon stored in Caribbean seagrass beds, the researchers used $18 per metric ton of carbon dioxide equivalents, borrowed from California’s cap and trade program. In addition to Caribbean-wide estimates, the researchers calculated values for individual countries in the region: The Bahamas has the largest share of Caribbean seagrass (61%), providing total ecosystem services valued at $156 billion annually, including $54 billion in carbon storage. Cuba ranks second in areal seagrass coverage (33% of the Caribbean total), with a value of $84.6 billion per year for all ecosystem services, including $29.3 billion for carbon storage. The dollar value of the carbon in seagrasses around Cuba is equivalent to 27% of the country’s 2020 GDP. “Importantly, the degradation of seagrass beds often leads to erosion and sediment resuspension, which can create a positive feedback of increased seagrass loss and the release of C stored in sediments,” the authors wrote. “Blue carbon finance thus represents a potential mechanism by which the global community can invest in conserving and protecting these vital ecosystems.” More than 60 species of seagrasses grow in shallow coastal waters around the world. They evolved from land plants that recolonized the oceans 70 to 100 million years ago. In a separate paper accepted for publication in the journal Proceedings of the Royal Society, Allgeier and colleagues show that the construction of artificial reefs in the Caribbean can help protect seagrass ecosystems from human impacts, including nutrient pollution and overfishing. Seagrasses use photosynthesis to pull carbon dioxide from the atmosphere, then store the carbon in plant tissues. The seagrasses are quickly inundated by sediments, slowing decomposition. As a result, more than 90% of the carbon stored in seagrass beds is in the top meter of sediment. Caribbean seagrasses and associated sediments store an estimated 1.3 billion metric tons of carbon, according to the new study. That’s a big number, but it’s just 1.09% of the carbon contained in above- and below-ground woody biomass in the Amazon, and just 1.12% of the carbon in the biomass and soils of the world’s temperate forests, according to the new
Study reveals widgeongrass has replaced eelgrass as the dominant seagrass species in Chesapeake Bay

Mangroves are growing in areas historically dominated by salt marshes and oyster reefs. Invasive pacific oysters are replacing native blue mussels in the Wadden Sea. Macroalgae are exhibiting dominance over hard corals in the Caribbean and Indo-Pacific. Climate change-driven shifts in dominant, habitat-forming species such as these can have significant implications for conservation, and this is a phenomenon that seems to be particularly prevalent for seagrass systems around the world. In research published today in the Proceedings of the National Academy of Sciences, VIMS researchers and their collaborators evaluate the causes and consequences of a new dominant seagrass species rising in the Chesapeake Bay, demonstrating both new threats and management opportunities. For this study, the authors combined 38 years of data on nutrient and sediment pollution from runoff, temperature, plankton blooms, and river flow with aerial seagrass surveys to describe the causes and consequences of shifting seagrass foundation species across 26,000 hectares of habitat in Chesapeake Bay. Demonstrated shifts in seagrass create faster recovery but larger die-offs across Chesapeake Bay Marine heatwaves and poor water clarity in Chesapeake Bay over the last few decades have cut the area occupied by the previously dominant seagrass, eelgrass, in half. At the same time, successfully implemented nutrient reductions throughout the bay have encouraged the rapid expansion of another seagrass, the cosmopolitan widgeongrass. Confined to a fringing area of shallow brackish waters until the mid 1990s, widgeongrass has now replaced eelgrass as the most abundant seagrass in Chesapeake Bay by expanding over 150% due to both high temperature-tolerance and long-lasting seeds that allow for rapid recovery after disturbance. “Widgeongrass’ recovery and expansion ability is so strong,” explains lead author Hensel, “that ideal widgeongrass conditions have fueled two record-setting peaks for Chesapeake Bay seagrass cover. In fact, much of the nearly 300% increase in Bay plants since the mid 1990s has been widgeongrass expansion into areas that eelgrass has vacated.” However, some negative consequences from the shift have concerned habitat managers. “We’ve seen periods of rapid widgeongrass expansion and retraction for decades now, far beyond what we’ve documented for eelgrass,” says Landry, co-author and leader of the Chesapeake Bay Program’s Submerged Aquatic Vegetation (SAV) Workgroup. “But now that widgeongrass has become so widespread in the bay, its fluctuating abundance can have a big impact on our overall acreage trends and on our Bay-wide restoration goal attainment. That’s the management concern. The ecological concern is the impact those fluctuations are having on the animals that have grown to depend on widgeongrass for habitat in the absence of eelgrass.” The causes of these fluctuations have been difficult to understand because widgeongrass and eelgrass appear to respond differently to climate change and nutrient pollution stressors. Yet when the authors examined long-term, large-scale data on climate stressors and watershed pollution from agriculture and development in tandem with year-to-year aerial survey imagery of seagrass meadows, they recognized an important shift in the dominant climate stressor for Chesapeake Bay seagrass: while widgeongrass is resistant to heatwaves and high temperatures, it is highly vulnerable to periods in spring when high rains can bring huge influxes of nutrient- and sediment-loaded water into the bay, reducing water clarity. “This study is an important step forward in building our knowledge of human-ecosystem interactions along the coast and how they are changing over time,” says author Lefcheck. “Our past work showed a record-setting resurgence of underwater grasses in response to nutrient management, but now we are seeing that the story is vastly more complex and in fact, is still being written. Understanding, adapting to, and communicating this shifting narrative is a challenge, but not an insurmountable one by any stretch.” Conserving species with different needs simultaneously is necessary, complex The team’s modeling contributes to a greater understanding of both human and climate drivers of annual changes in widgeongrass and highlights a crucial difference in management compared to an eelgrass-dominated bay. “Heatwave stress is uncontrollable on a local and regional level,” explains Patrick, Director of the SAV Monitoring Program at VIMS, “but managing the amount of nutrients that enter the bay from the watershed during a rainy spring is something that we can actually control.” This study underscores that, because species differ in their traits and stressor sensitivities, managing for conservation of living habitats requires more community- or species-focused research, monitoring, and actions under climate change. Climate change is shifting the landscape of species composition, creating new winners in these novel environments, and management needs to shift alongside them. Detailed monitoring data allow agencies to focus on each species and encourages them to manage for community or individual habitat requirements, adapting strategies as species composition changes. This study also demonstrates pitfalls that can occur if habitat forming species are lumped together into single “stocks,” such as “hectares of seagrass” or “acres of marsh.” In fact, differences between the seagrass species in this study explain many of the shifts in Chesapeake Bay seagrass meadow dynamics. “Widgeongrass has shorter, thinner blades than eelgrass,” says Hensel, “which makes it more vulnerable to springtime run-off events because sunlight can’t reach the short blades through the clouded, nutrient-loaded water. Also, widgeongrass’ shallower root system may not sequester carbon as well and its tendency to wildly fluctuate in cover means that it may not provide consistent habitat for key seagrass-dependent species like Blue Crabs and Black Sea Bass.” The authors call for a parallel shift in coastal monitoring and evaluation of management successes and failures, using the Chesapeake Bay as an example. They cite the necessity of long-term monitoring programs with coordinated and standardized on-the-ground and detail-oriented surveys, as well as the importance of community or species-specific recovery goals across coastlines where multiple seagrass species co-occur and respond to climate differently. “This is a compelling example of the how climate change is unfolding across the globe,” said Patrick. “As regional climates shift, the emergence of novel ecosystems is fundamentally challenging everything we think we know from analysis of historical data. The rules governing the dynamics of the world’s ecosystems are changing and
What causes decline of tropical seagrass meadows?
Seagrass, a group of aquatic angiosperms, grows in shallow waters in the coastal sea and contributes most of the primary production while participating in many important ecological processes. Heat stress threatens the survival of seagrass, but its damage mechanisms are unclear. Recently, a research team led by Prof. Liu Jianguo from the Institute of Oceanology of the Chinese Academy of Sciences (IOCAS) primarily explained the physiological and biochemical mechanisms underlying the decline of tropical seagrass meadows caused by heat stress combined with high light at different functional levels. The study was published in Marine Pollution Bulletin. During the lowest tide, intertidal seagrass is frequently faced with the combined stress of exposure to air, direct sunlight, and high temperature, which may have a strongly negative impact on the survival of seagrass, especially in the context of global warming. The researchers found that the largest tropical seagrass Enhalus acoroides can withstand heat below 39°C in the dark. However, under high light, the tolerance to heat stress is greatly reduced. The combined effect of short-term exposure to heat and high light stress destroyed the photosystem II (PSII) and destroyed key components of the photosynthetic system in seagrass leaves. Moreover, high light combined with heat stress caused severe oxidative stress in the seagrass, which led to irreversible damage to the seagrass. These results clearly suggest that heat stress coupled with high light, may be an important cause for the decline of E. acoroides meadows. “Our study reveals that ocean warming, especially when coupled with high light, exacerbates the decline of seagrass meadows and affects the ecological function of intertidal seagrass meadows,” said Dr. Zhang Mengjie, first author of the study. “This serves as a warning that the effects of global warming on seagrass meadows will be even worse than expected,” said Prof. Liu, corresponding author of the study. More information: Mengjie Zhang et al, Heat stress, especially when coupled with high light, accelerates the decline of tropical seagrass (Enhalus acoroides) meadows, Marine Pollution Bulletin (2023). DOI: 10.1016/j.marpolbul.2023.115043

